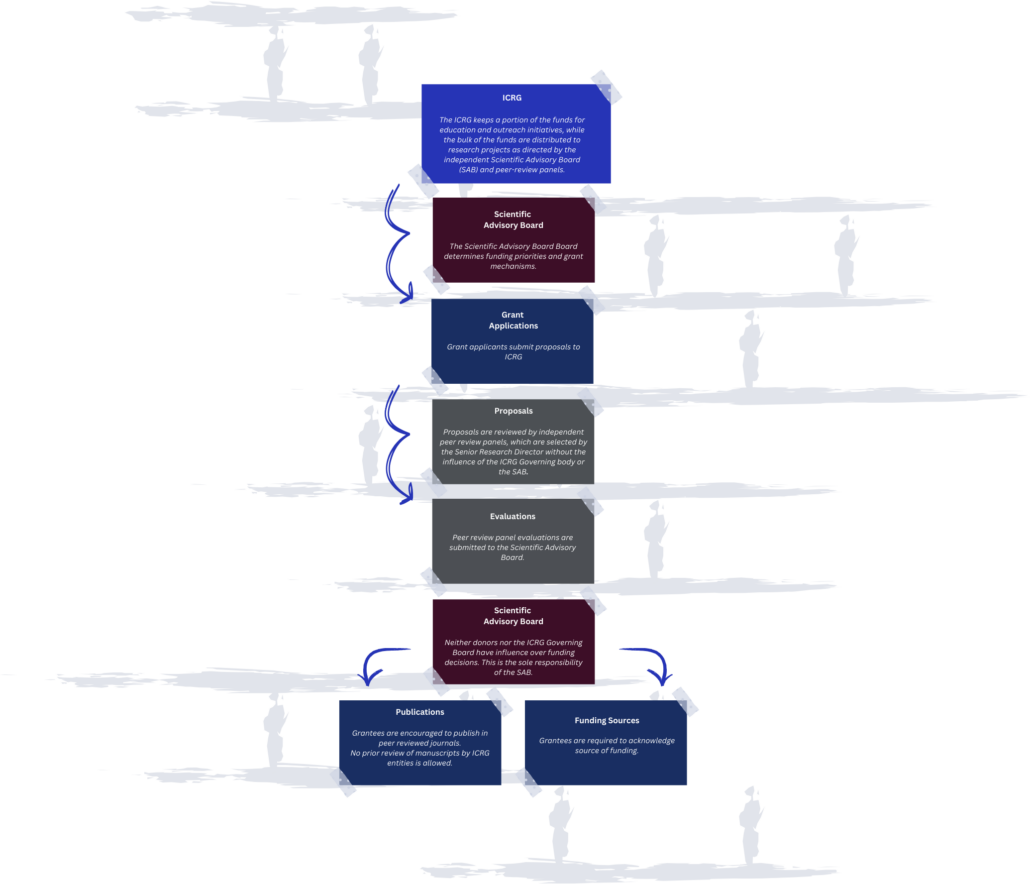
ICRG Firewall
Grant Review Criteria
The ICRG seeks proposals of high scientific merit from investigators who show promise of disseminating their work at high-impact conferences and in peer-reviewed scientific journals. Proposals are evaluated by peer-review panels of experienced researchers in the field of addictions according to the following criteria, which are adapted from the National Institutes of Health:
- Significance. Does the project address an important problem or a critical barrier to progress in the field? If the aims of the project are achieved, how will scientific knowledge, technical capability and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services or preventative interventions that drive this field?
- Investigator(s). Are the principal investigator, collaborators and other researchers well suited to the project? If Early-Stage Investigators, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
- Innovation. Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation or interventions? Are the concepts, approaches or methodologies, instrumentation or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement or new application of theoretical concepts, approaches or methodologies, instrumentation or interventions proposed?
- Approach. Are the overall strategy, methodology and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Are potential problems, alternative strategies and benchmarks for success presented? If the project involves clinical research, are the plans for protection of human subjects from research risks justified in terms of the scientific goals and research strategy proposed?
- Environment. Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations or collaborative arrangements?
Peer Reviewers
The following scientists reviewed grants applications for the ICRG in 2023:
Brian Borsari, PhD
Professor of Psychiatry
University of California-San Francisco Weill Institute for Neurosciences
Bethany Bray, PhD
Associate Professor
Department of Medicine
University of Illinois at Chicago
Luke Clark, PhD
Professor of Psychology
Director of the Centre for Gambling Research
University of British Columbia
Heather Gray, PhD
Assistant Professor, Harvard Medical School
Director of Academic Affairs at the Division on Addiction
Cambridge Health Alliance
Joshua Grubbs, PhD
Associate Professor
Bowling Green State University
Scott Huettel, PhD
Professor in the Department of Psychology and Neuroscience
Bass Fellow
Professor of Neurobiology
Duke University
Debi LaPlante, PhD
Associate Professor of Psychiatry, Harvard Medical School
Director, Division on Addiction, Cambridge Health Alliance
Matthew Martens, PhD
Associate Provost for Academic Programs
University of Missouri, Columbia
Dipali Rinker, PhD
Assistant Professor
Behavioral Sciences and Population Health
American University of the Caribbean School of Medicine
Paul Sacco, PhD
Associate Professor of Social Work
University of Maryland, Baltimore
Nathan Smith, PhD
Executive Director
Kindbridge Research Institute
Krishna Vaddiparti, PhD
Assistant Professor of Epidemiology
University of Florida
Jeremiah Weinstock, PhD
Professor, Clinical Program
Department of Psychology
Saint Louis University
ICRG Policy on Academic Integrity and Research Misconduct
“Research misconduct is defined as fabrication, falsification and plagiarism, and does not include honest error or differences of opinion.” (ORI 2005)
ICRG is committed to ensuring the academic integrity of all research funded with its grants. ICRG conducts multiple reviews of grant applications and related proposals every year. Peer reviewers who believe they have identified research misconduct in the form of fabrication, falsification or plagiarism are required to alert ICRG’s Senior Research Director immediately upon such concern. The allegation must not be discussed during peer review, and the reviewer making the allegation will be reminded of the ICRG policy on confidentiality. An application flagged for possible misconduct will still be put through the peer review process.
Within 30 days of being alerted to possible misconduct, the Senior Research Director will convene a meeting of the Scientific Advisory Board (SAB) to discuss the allegation. The Senior Research Director will be required to assess any conflicts of interest within the SAB prior to the meeting.
Within 30 days of such meeting, the SAB will decide by at least a two-thirds/majority vote of all of its then members if the alleged misconduct should be reported to the grant applicant’s institutional office of research integrity. Alleged misconduct must be deemed egregious in the eyes of the SAB to be so reported. The Senior Research Director will then be solely responsible for any communications with the applicant’s institution on behalf of ICRG.
NIH Office of Scientific Integrity Definitions for purposes of this Policy: Fabrication: Making up data or results and recording or reporting them. Falsification: Manipulating research materials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented Plagiarism: The appropriation of another’s ideas, processes, results, or words without giving appropriate credit
References:
Handling Misconduct | ORI – The Office of Research Integrity. (2005). The Office of Research Integrity. https://ori.hhs.gov/handling-misconduct
Eisner, R., & Vasgird, D. (2003). Responsible Conduct of Research : Research Misconduct. https://Ccnmtl.Columbia.Edu/Projects/Rcr/Rcr_misconduct/WinResources.Html.

